|
 The program to study the deep sea gas hydrate deposits is led by the Japan Oil, Gas and Metals National Corporation (JOGMEC) and Japan’s National Institute of Advanced Industrial Science and Technology (AIST). The project is being conducted in collaboration with the United States Geological Survey (USGS) Gas Hydrates Project and researchers from the School of Civil and Environmental Engineering at Georgia Tech. This project is one component of an ongoing Japanese collaboration on methane hydrate research with the United States Department of Energy (DOE) and the Gulf of Mexico Gas Hydrate Joint Industry Project (JIP). The program to study the deep sea gas hydrate deposits is led by the Japan Oil, Gas and Metals National Corporation (JOGMEC) and Japan’s National Institute of Advanced Industrial Science and Technology (AIST). The project is being conducted in collaboration with the United States Geological Survey (USGS) Gas Hydrates Project and researchers from the School of Civil and Environmental Engineering at Georgia Tech. This project is one component of an ongoing Japanese collaboration on methane hydrate research with the United States Department of Energy (DOE) and the Gulf of Mexico Gas Hydrate Joint Industry Project (JIP).
The research team accomplished the technically difficult goal of recovering cores from the gas hydrate deposits for laboratory testing. Well-preserved samples are extremely rare. They are preserved as “pressure cores,” with the gas hydrates kept as if they were still at the natural conditions in the subsurface where they formed. Gas hydrates are only stable at certain pressures and temperatures, and scientists have been working since the 1990s on sophisticated techniques to retrieve and preserve samples. The cores must be maintained under high pressures and low temperatures to prevent the hydrates from dissociating.
What are Gas Hydrates?
Gas hydrate, methane hydrate, or methane clathrate (CH 4•5.75H 2 O), is a naturally-occurring, solid form of methane gas combined with water molecules. The solid material is an ice-like substance formed when methane combines with water under specific pressure and temperature conditions. Gas hydrates can also be formed with other gases such as ethane, hydrogen sulfide, or carbon dioxide.
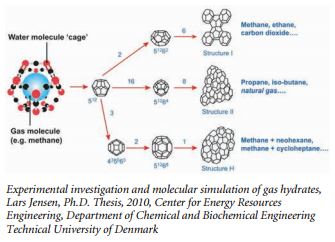
According the USGS Hydrates Primer (http://woodshole.er.usgs. gov/project-pages/hydrates/primer.html), at the molecular level, gas hydrate consists of gas molecules surrounded by cages of water molecules. Each water cage encloses a space of a particular size, and only a gas molecule small enough to fit within this site can be hosted in that specific hydrate structure. Clathrate hydrates are not chemical compounds, as the sequestered molecules are never bonded to the lattice. Structure I gas hydrate has 46 water molecules that enclose 8 sites where gas molecules may be hosted. Six larger gas sites are enclosed by water cages with 12 pentagonal and 2 hexagonal faces, while two smaller gas sites occur within pentagonal dodecahedral cavities. Without the support of the trapped molecules, the lattice structure of hydrate clathrates would collapse into conventional ice crystal structure or liquid water. Some researchers have likened the water cage structures to buckyballs.
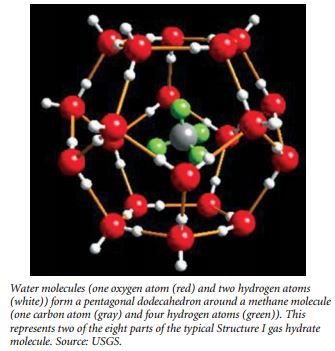 Methane molecules can fit within both the small and large sites in the Structure I lattice, the most common type found in nature. Thus, “gas hydrate” and “methane hydrate” are often used interchangeably by researchers. Methane molecules can fit within both the small and large sites in the Structure I lattice, the most common type found in nature. Thus, “gas hydrate” and “methane hydrate” are often used interchangeably by researchers.
Methane hydrate takes many forms in sediments. In finegrained sediments, the methane hydrate can form in small pores and cement the grains, but may not be visible. Gas hydrate has also been recovered in chunks, in veins within sediments, and occasionally in large masses. According to the report “Energy Resource Potential of Methane Hydrate” dated February 2011 by the U.S. Department of Energy’s National Energy Technology Laboratory, methane hydrate is a fairly concentrated form of natural gas. When dissociated at standard temperature and pressure, one cubic foot of solid hydrates will yield about 164 cubic feet of methane gas.
Gas Hydrate Occurrence and Distribution
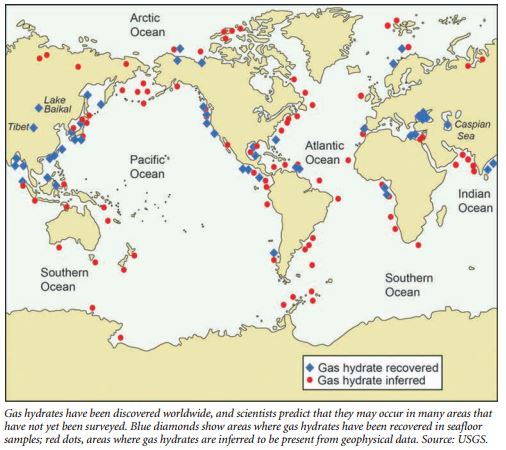
Gas hydrates are widespread and found in large volume in marine sediments at, and beneath, the ocean floor and in sediments within and beneath permafrost areas. These pressure-temperature conditions keep the gas hydrate “stable,” meaning the gases are contained in its solid form.
While gas hydrates can sometimes appear as clumps on the seafloor, researchers are most interested in the hydrates that form within sediments for potential energy production. Within the sediments, the source of the methane can be either microbial or from deeper thermogenic sources. The methane gas migrates upward until it mixes with water under specific conditions of temperature and pressure. Most of the gas hydrate occurs in the uppermost hundreds of feet of sediments at ocean water depths greater than approximately 1,500 feet and close to continental margins.
The spatial association of gas hydrates along continental margins is related to the availability of ample organic carbon from terrestrial sources or generated within the water column and incorporated into the sediments. Microbes use this carbon to generate methane. Microbial generation is the most common source for the methane observed in the studied natural gas hydrates. These observations may be biased by the relatively shallow sub-seafloor depths from which most of gas hydrate samples have been recovered.
Prior to 1995, there had been no dedicated drilling of gas hydratebearing deposits, and estimates of the amount of methane in the gas hydrates ranged widely. Conventional natural gas volumes are most often cited in units of trillions of cubic feet (TCF). Most studies published in the past 15 years have concluded that between 100,000 and 5,000,000 TCF of methane is trapped in global gas hydrate deposits. While the low-end estimate is more than 4000 times the amount of natural gas consumed annually in the United States, only a fraction of the methane sequestered in global gas hydrate deposits is likely to be concentrated enough and accessible enough to ever be considered a potential target for energy resource studies.
 Potential Development Potential Development
The sedimentary methane hydrate reservoir probably contains two to ten times the currently known reserves of conventional natural gas. This represents a potentially important future source of energy. However, in the majority of sites deposits are thought to be too dispersed for economic extraction. Other problems facing commercial exploitation are detection of viable reserves and development of the technology for extracting methane gas from the hydrate deposits.
Current studies on the potential development of gas hydrate resources are focusing on better understanding on several key factors likely to affect success. Besides investigating the porosity and permeability in various hydrate-bearing sediments, laboratory studies are performed on cores to measure the sediments’ thermal conductivity, that is, how quickly heat will flow through them. Additional areas of study are the mechanical properties like strength and stiffness of the sediment, both with hydrates and after the hydrates dissociate and the gas and water are removed.
The aim of the ongoing research is to develop innovative methods and gas recovery techniques, both for longer term tests and for eventual commercial production. For now, hydrate production calls for using conventional methods in which a well is drilled into sediments, lined with casing and kept filled with water. The water would be pumped out, lowering the pressure enough so the hydrates dissociate. Because water is produced along with the gas, the pumping would have to be continuous.
But the dissociation of hydrates is endothermic — it uses, rather than releases, energy — so when methane gas is produced, the sediments begin to cool. The cooling can slow or stop dissociation. To compensate for this production will probably have to involve the introduction of heat as well as pumping.
Conventional techniques would not work well in clays, which contain the vast majority of known hydrate reserves, because of the low permeabilities. In the research project headed by JOGMEC, where natural gas was extracted, specialized equipment was used to drill into and depressurize the hydrate deposits, causing the methane to separate from the ice. The gas was then collected and piped to surface.
In a September 2013 article in the New York Timesby Henry Fountain, Dr. Carlos Santamarina, a professor at Georgia Tech, said, “Much of the current paradigm for production in methane hydrates is anchored around oil production. And probably with that paradigm we may not go very far.” Despite the technical challenges, the potential for gas hydrates is huge.
|