|
The Gas and Its Physical Properties
Helium is the second lightest and the second most abundant element in the observable universe where it makes up about 24% of the total elemental mass. This mass is more than 12 times greater than the mass of all the heavier elements combined. Most helium in the universe is helium-4 and is believed to have been created in the Big Bang and during the fusion reactions in stars.
Despite this abundance, helium, the lightest of the noble gases, is rare in the Earth’s atmosphere making up only approximately 0.00052% of the volume or 5.2 parts per million. In seawater, the concentration is only 4 parts per trillion. The atmospheric concentration is fairly constant despite the continuous release of helium from the lithosphere because most helium in the Earth’s atmosphere migrates upward and finally escapes into space. In the Earth’s heterosphere, the atmospheric zone more than 60 miles above the ground surface where gases separate by molecular weight, helium and other lighter elements are the most abundant gases.
At standard temperature and pressure, helium is a colorless, odorless, non-toxic, inert gas in the noble gas group. The boiling point of helium, -452° F (-269° C), is the lowest among the elements. This means that helium does not become a liquid until just a few degrees above absolute zero. A chemist or a physicist will tell you that the “nuclear binding energy” of helium-4 is very high which is why it is a product of both nuclear fusion and radioactive decay.

Helium, named for the Greek god Helios, was first detected in 1868 by the French astronomer Jules Janssen and the British physicist Norman Lockyer in the spectra from a solar eclipse. In 1895, helium was formally discovered on Earth by the Swedish chemists, Per Teodor Cleve and Nils Abraham Langlet. The Swedes observed the gas emanating from the uranium ore cleveite.
Geological Origin
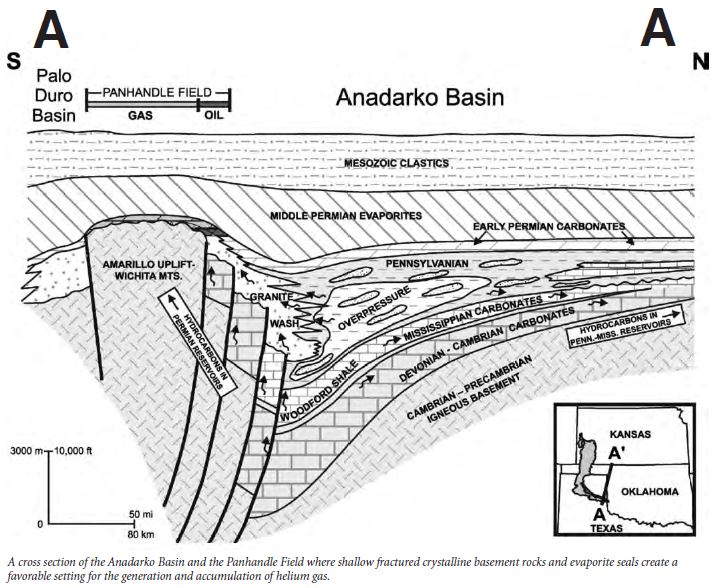
Most terrestrial helium was created by the natural decay of heavy radioactive elements, principally thorium and uranium, in the minerals cleveite, pitchblend (uraninite), carnotite, and monzanite. These minerals are often found in granitoid basement rocks. The decay of these elements emits alpha particles which consist of two neutrons and two protons. Alpha particles are the same as helium nuclei. The nuclei pick up electrons from the surrounding rock. These processes are estimated to generate approximately 3,300 tons of helium per year in the Earth’s crust where the concentrations are up to eight parts per billion.
 Because helium migrates and is trapped in the subsurface under conditions that also trap hydrocarbons, the greatest natural concentrations of helium on the planet are found in natural gas. Most unprocessed natural gas contains at least trace amounts of helium, yet very few natural gas fields contain commercially exploitable concentrations. Helium concentrations in natural gas vary over a wide range from a few parts per million to greater than 70,000 parts per million (7 percent). Natural gas must contain at least 0.3% helium to be considered as a potential helium resource. Because helium migrates and is trapped in the subsurface under conditions that also trap hydrocarbons, the greatest natural concentrations of helium on the planet are found in natural gas. Most unprocessed natural gas contains at least trace amounts of helium, yet very few natural gas fields contain commercially exploitable concentrations. Helium concentrations in natural gas vary over a wide range from a few parts per million to greater than 70,000 parts per million (7 percent). Natural gas must contain at least 0.3% helium to be considered as a potential helium resource.
The wide range of helium concentrations in natural gas is determined by the geological setting. The geological setting most favorable for the accumulation of higher concentrations of helium involves the juxtaposition of three conditions:
1) Granitoid basement rocks rich in uranium and thorium at a relatively shallow depth;
2) Fractured and faulted basement rocks providing a pathway for the helium to escape; and
3) An overlying trap consisting of a porous reservoir of sedimentary rocks capped by an impermeable seal.
The nature of the seal is critical for the accumulation of helium gas in exploitable concentrations. Helium has the smallest atomic radius of any element, about 0.2 nanometers. As it migrates upward through the stratigraphic column, the gas can fit through very small pore spaces. Typically, halite or anhydrite are the only sedimentary rocks that can block the upward migration of helium atoms. In some cases, shales with pore spaces plugged with abundant organic materials (kerogen) can serve as a less effective barrier.
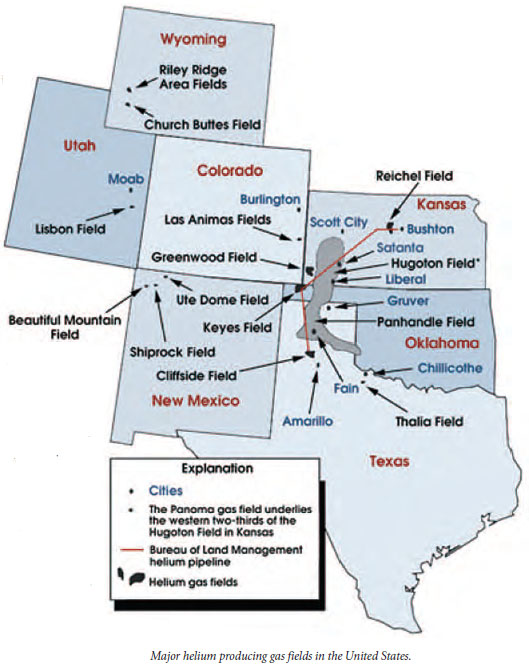
By 1903, large reserves of helium were identified in natural gas fields in Oklahoma, Texas, and New Mexico. The Hugoton (Kansas, Oklahoma, and Texas), Panhandle West, Panoma, Riley Ridge in Wyoming, and Cliffside Fields are the gas fields where most domestically-produced helium has been extracted in the last 75 years.
The natural gas fields in northwest New Mexico have some of the highest known helium concentrations. These elevated levels of helium in natural gases occur in Paleozoic reservoirs on the Four Corners Platform and also in Paleozoic reservoirs in the deeper parts of the San Juan Basin. According to a 2012 publication by R.F. Broadent titled Helium in New Mexico: Origins, Uses, Economics, Geologic Distribution and Exploration Possibilities, the regional set of orthogonal faults that offset Precambrian basement throughout the deeper parts of the San Juan Basin may have acted as migration pathways that transmitted helium from its basement source into overlying Paleozoic reservoirs.
 Helium Production and Storage Helium Production and Storage
Helium is commercially extracted from natural gas by a low temperature separation process called fractional distillation. Since helium has a lower boiling point than any other element, low temperature and high pressure are used to liquefy and draw off nearly all the other gases (mostly nitrogen and methane). The resulting crude helium gas (50 to 70 percent helium) is purified by successive exposures to lowering temperatures, in which almost all of the remaining nitrogen and other gases are precipi tated out of the gaseous mixture. Activated charcoal is used as a final purification step, usually resulting in 99.995% pure Grade-A helium. The principal impurity in Grade-A helium is neon. In a final production step, most of the helium that is produced is liquefied via a cryogenic process. This is necessary for applications requiring liquid helium and also allows helium suppliers to reduce the cost of long distance transportation.
The United States began stockpiling helium after World War I because Congress was concerned about catching up with the Germans in the race to build a fleet of dirigibles. In 1925, the United States established the National Helium Reserve, also known as the Cliffside Field Government Reserve, near Amarillo, Texas to serve as a strategic supply of helium for use in airships and other defense purposes.
The Bureau of Mines constructed and operated the large helium extraction and storage facility that went into operation in 1929. From 1929 to 1960 the federal government was the only domestic producer of helium. Although the use of airships declined after World War II, new defense uses for helium arose such as a purging gas when refueling rocket engines and as a coolant in nuclear weapons programs.
As of December 2006, the total helium reserves and resources of the United States were estimated to be 744 billion cubic feet (BCF). This included 153.2 BCF of measured reserves, 192.2 BCF of probable resources, 213.8 BCF of possible resources, and 184.4 BCF of speculative resources. Included in the measured reserves were 24.2 BCF of helium stored in the National Helium Reserve. Helium resources outside of the United States are estimated to be approximately 1.13 trillion cubic feet. The majority of these resources are located in Qatar (362 BCF), Algeria (294 BCF), Russia (244 BCF), Canada (72 BCF), and China (40 BCF).
The National Helium Reserve is now operated by the Bureau of Land Management (BLM), a branch of the Department of the Interior, under the Federal Helium Program (Public Law 104-273). The Federal Helium Program includes all operations of the Bush Dome and Cliffside Field storage reservoirs in Potter County, Texas and the crude helium pipeline system. The BLM no longer supplies Federal agencies with Grade-A helium. Private firms that sell Grade-A helium to Federal agencies are required to purchase a like amount of (in-kind) crude helium from the BLM.
Usage and Price
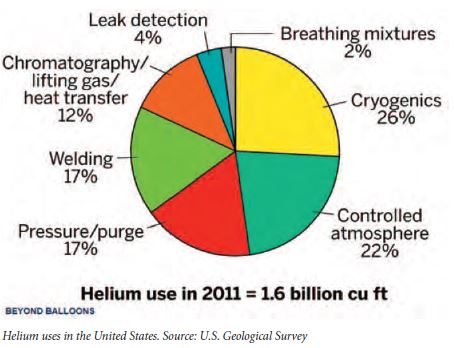
Due to its properties, helium plays a unique role in many commercial applications. Helium is used extensively in cryogenic applications, particularly in the cooling of superconducting magnets. The main commercial application for superconducting magnets is in medical equipment, particularly in magnetic resonance imaging scanners. There is no substitute for helium in cryogenic applications if temperatures below –429 °F are required. Cryogenic uses consume approximately one quarter of the United States production. Helium’s other industrial uses, accounting for approximately 50 percent of production, include: a pressurizing and purge gas, protective atmosphere for arc welding, and in processes such as growing crystals to make silicon wafers. The most well-known use is as a lifting gas in balloons and airships.
In 1995, Congress decided that the National Helium Reserve was not essential and initiated a program to sell the stored helium as part of the Helium Privatization Act of 1996 (Public Law 104–273). In accordance with the Act, the BLM began selling off the gas to private companies, while keeping 600 million cubic feet in reserve. The liquidation of the reserve had the aim of reducing the debt incurred by the program, but the sales also artificially depressed the price of helium.
Since then, up to one half of the world’s helium demand has been met through sales from the reserve and, in some years, more helium was exported out of the United States for use overseas than was consumed domestically. Many analysts believe that the dumping of National Helium Reserve supplies onto the market has depressed the price of helium so much that it is being used as a cheap substitute for argon and other gases that have a much less limited supply, and thus is being wasted.
According to the January 2013 United States Geological Survey Mineral Commodity Summary estimated domestic consumption of helium gas in 2012 was 1.8 BCF. The price for crude helium in fiscal year 2012 was $65.50 per thousand cubic feet to government users and $75.75 per thousand cubic feet to nongovernment users. The price for the government-owned helium is mandated by the Helium Privatization Act. At the same time, the estimated price Grade-A gaseous helium from private industry sources was about $170 per thousand cubic feet. Many argued that the government was not getting a fair price for the helium.
Amid the rising concern related to the closing of the Federal Helium Reserve, the House of Representatives passed bipartisan legislation on September 26, 2013 to postpone the government mandated shutdown in October 2013. President Obama signed the Responsible Helium Administration and Stewardship Act of 2013 into law which prevented the premature closing of the reserve.
 The Future The Future
Although helium is continually being produced by radioactive mineral decay in Earth’s crust, the rate of natural production and accumulation is so low that the gas can be considered a nonrenewable resource. Some analysts estimate that, based on the forecasted rated of consumption and the volume of the known reserves, the helium supply will be depleted in about 40 years.
The commercial development of helium production has been stymied by the government-mandated price during the liquidation of the reserve. Industrial consumers are concerned that the market will be undersupplied when National Helium Reserve sales are discontinued. When that occurs, free market forces could lift the price of helium by an order of magnitude as commercial capacity will be inadequate to meet demand. As the helium marketplace shifts from one dominated by distributions from the government reserve to a true commodity, the price will surely rise.
Producing helium is capital and energy intensive. Energy costs strongly affect the cost to refine helium from natural gas. A large investment is needed in facilities, storage, and pipelines for commercial scale helium production. With the market price of helium in flux, commercial interests are reluctant to make the required investments. But with forecasts for rising prices and potential shortages, new helium production plants are being built outside the United States.
Domestic suppliers are making plans to construct facilities to meet the future demand of industrial users. Geologists will play an important role in identifying geological settings favorable for the generation and accumulation of commercial concentrations of helium in natural gas reservoirs. So, while the exploration for natural gas will continue to draw most of the attention of a large number of geologists, some of them will be on the lookout for helium, “the other natural gas.”
|

 Helium: The Other Natural Gas
Helium: The Other Natural Gas
 Because helium migrates and is trapped in the subsurface under conditions that also trap hydrocarbons, the greatest natural concentrations of helium on the planet are found in natural gas. Most unprocessed natural gas contains at least trace amounts of helium, yet very few natural gas fields contain commercially exploitable concentrations. Helium concentrations in natural gas vary over a wide range from a few parts per million to greater than 70,000 parts per million (7 percent). Natural gas must contain at least 0.3% helium to be considered as a potential helium resource.
Because helium migrates and is trapped in the subsurface under conditions that also trap hydrocarbons, the greatest natural concentrations of helium on the planet are found in natural gas. Most unprocessed natural gas contains at least trace amounts of helium, yet very few natural gas fields contain commercially exploitable concentrations. Helium concentrations in natural gas vary over a wide range from a few parts per million to greater than 70,000 parts per million (7 percent). Natural gas must contain at least 0.3% helium to be considered as a potential helium resource.
 Helium Production and Storage
Helium Production and Storage The Future
The Future